35+ calculating ph from ionic strength
1 p H log 10 H. Ionic compounds when dissolved in water dissociate into ions.

Calculated Ionic Strength And Ph Of The Feedwater At Different Download Scientific Diagram
Web Im having trouble interpreting the data for an experiment.
. 35 Mixed acid dissociation constants. Web a change in ionic strength affects the dissociation of acids. Using activities calculate the pH of.
Enter the concentration and valency separated by a comma in the respective input field. Web Question Use activities to calculate the pH of each of the following solutions being sure to use mathrmalpha values and the extended Debye-Huckel equation. With increasing ionic strength I found lower solubility but also lower pH of the solution.
Web First step in calculations is calculation of so called ionic strength using the following formula. Web Ionic strength is typically calculated as the product of a given ions concentration ci and its charge zi summed over all ions in solution divided by two. K Cl- H 3O OH-.
This activity coefficient is used in the calculation of the pH s saturation pH but it can also be. In a low ionic strength liquid this reaction can be small and. Solution First determine what ions are present in solution.
People have written computer programs to calculate. Where C i is a molar concentration of i th ion present in the solution and z i. Web The procedure to use the ionic strength calculator is as follows.
Web The ionic strength of a solution is a measure of the concentration of ions in that solution. Web Calculate the ionic strength of a 0060 M solution of KCl. Web The reason is that Mg 2 ions and H ions bind to ATP and the equilibrium constants are affected by the pH.
Know the steps to calculate the ionic strength of a chemical solution on this pageThis handy calculator does all the lengthy calculations and gives. Web PH probes work by the concentration of hydrogen ions creating a milivolt output that the probes calculate a pH from. Web Ionic Strength Calculator.
The practical alter- native Many elementary biochemical textbooks include a. Web Students of chemistry are well acquainted with the relationship between solution pH and hydrogen-ion concentration. Web The activity coefficient is calculated with formula 2.

Ph And Ionic Strength Effects On Cation Concentrations Mg L 1 Download Table

Ph Calculations And More In Fundamentals Of Pharmaceutics 07 27 13
How To Calculate An Ionic Strength Of A Buffer Solution Sciencing
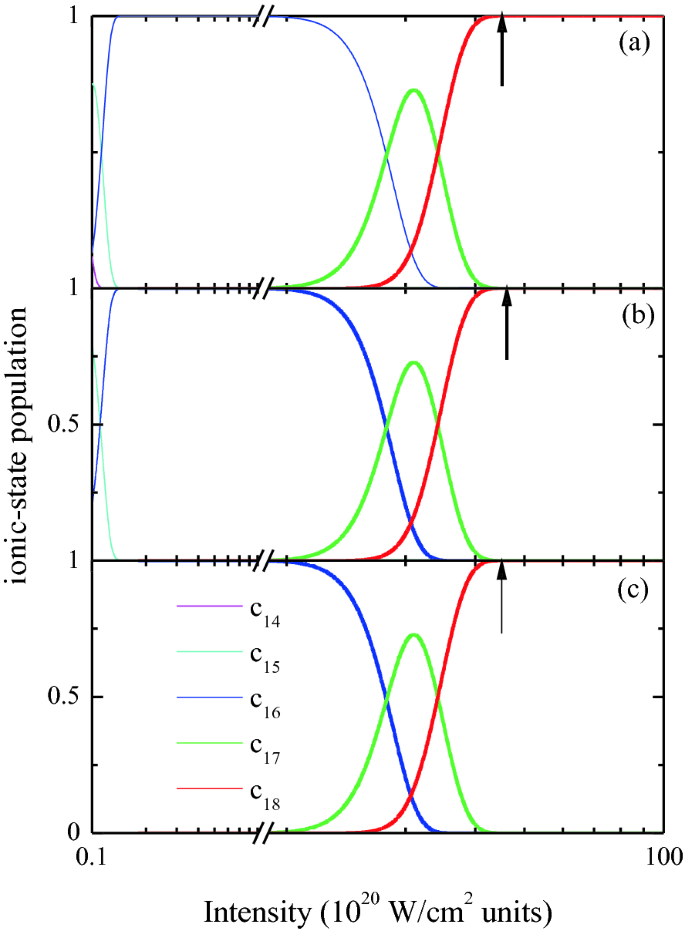
Towards Laser Intensity Calibration Using High Field Ionization Springerlink
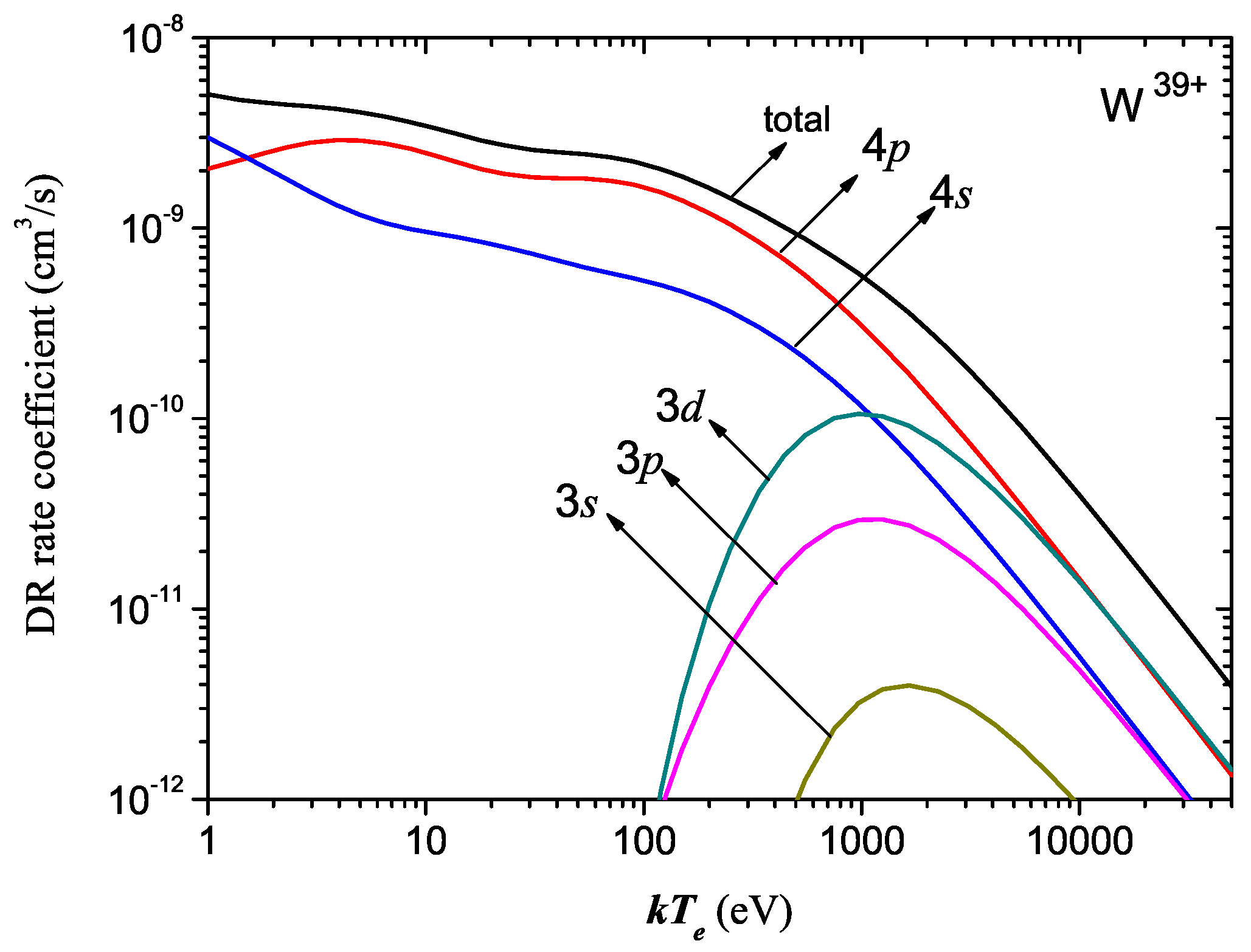
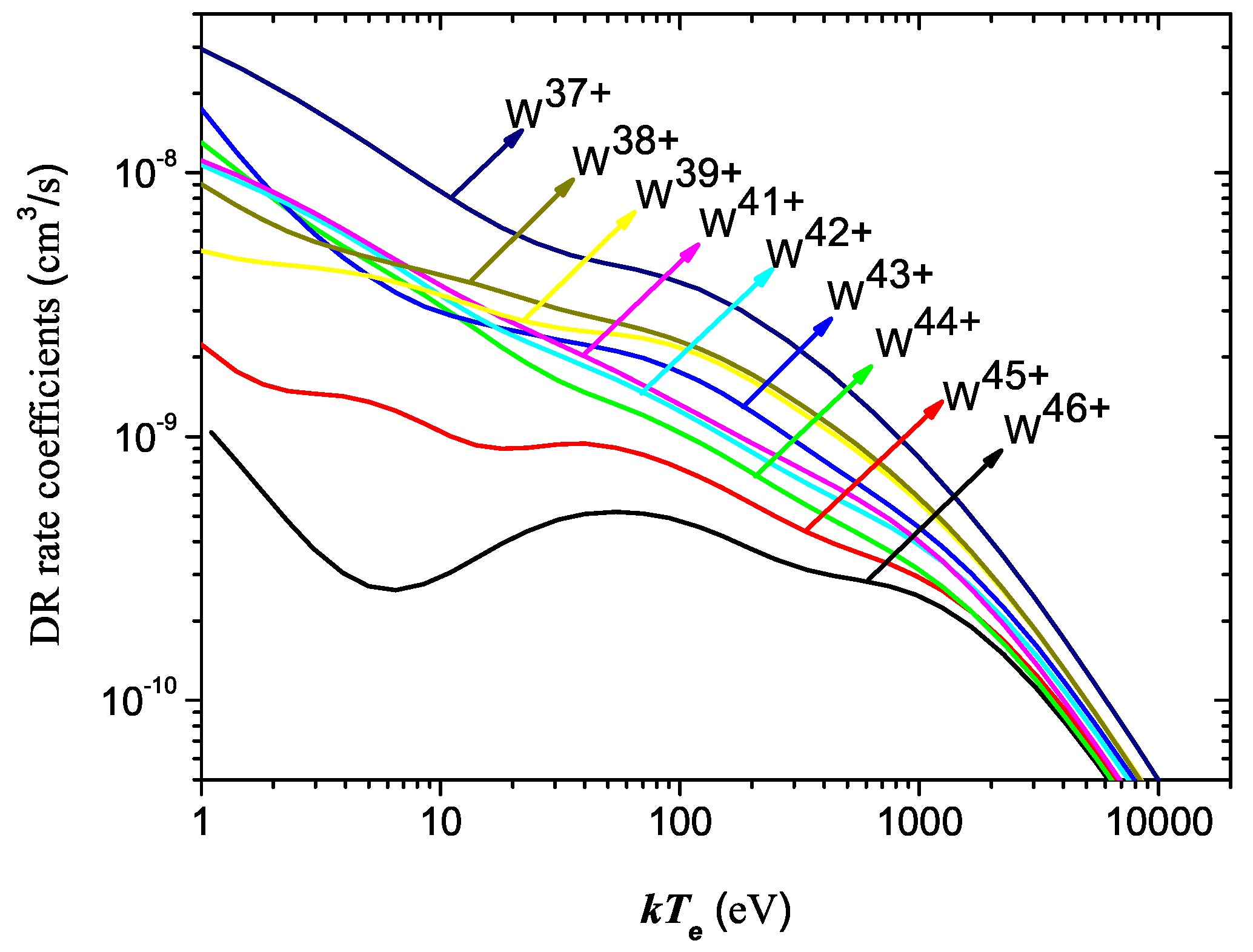
Atoms Free Full Text Electron Impact Excitation And Dielectronic Recombination Of Highly Charged Tungsten Ions
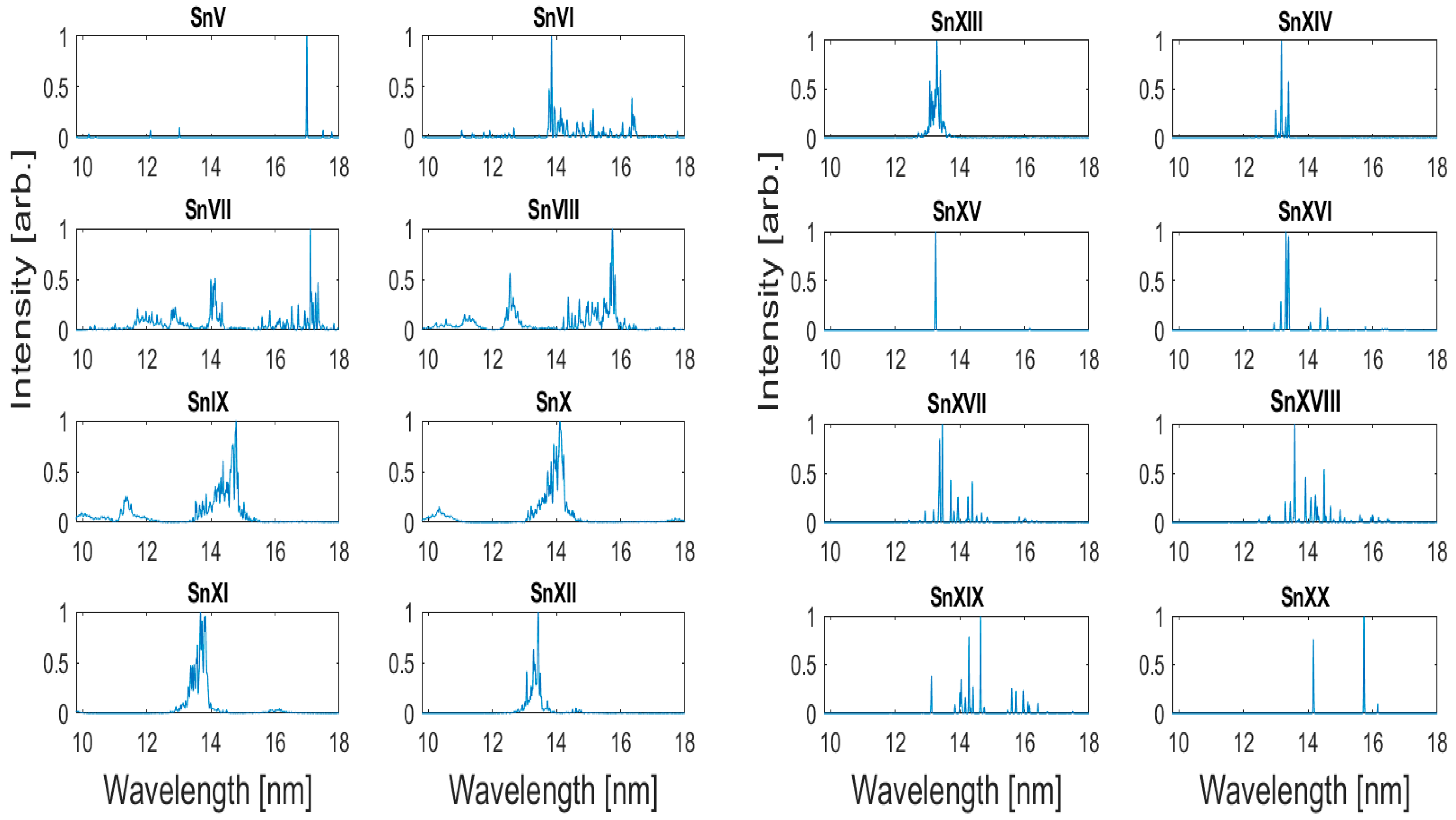
Atoms Free Full Text An Investigation Of Laser Produced Lead Tin Alloy Plasmas Between 10 And 18 Nm
Why Would The Blood Ph Increase In Hyperammonemia When Ammonium Ions In Blood Are Conjugate Acid Of Ammonia With Pka Of 9 4 Quora

Affinity Of The Highly Preorganized Ligand Pda 1 10 Phenanthroline 2 9 Dicarboxylic Acid For Large Metal Ions Of Higher Charge A Crystallographic And Thermodynamic Study Of Pda Complexes Of Thorium Iv And The Uranyl Vi Ion Inorganic

Sustainability Performance Of Polyethylene Terephthalate Clarifying Challenges And Opportunities Sarda 2022 Journal Of Polymer Science Wiley Online Library

Buffers With Specific Ionic Strength 1 How Many Ml Of 12 0 M Acetic Acid And How Many Grams Of Sodium Acetate Fw 82 G Mol Are Needed To Prepare A Ppt Download

How Do I Calculate The Ph Dependent Ionic Strength Of A Phosphate Buffer Na Dibasic Heptahydrate Na Monobasic Dihydrate Researchgate

True Or False As Ionic Strength Increases So Does The Ph Of Pure Water Homework Study Com

How To Calculate An Ionic Strength Of A Buffer Solution Sciencing

Entering The Sample Preparation Acronym Maze

Igcse Chemistry Revision Guide 2012

Interaction Of Slow Very Highly Charged Ions With Surfaces Sciencedirect

Chain Shattering Polymers As Degradable Microdispersive Solid Phase Extraction Sorbents Analytical Chemistry